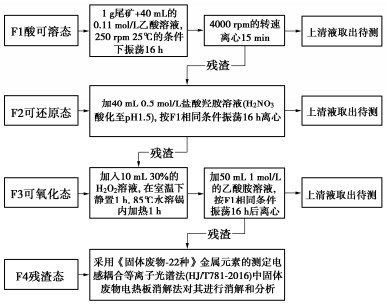
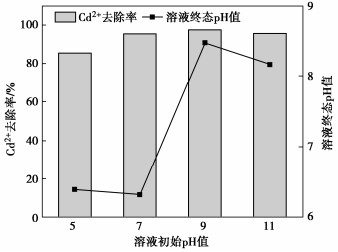
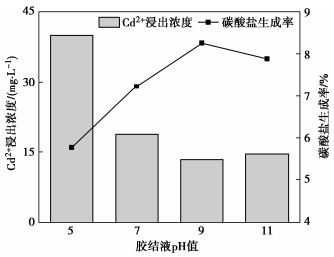
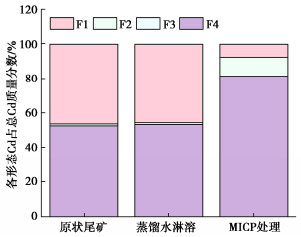
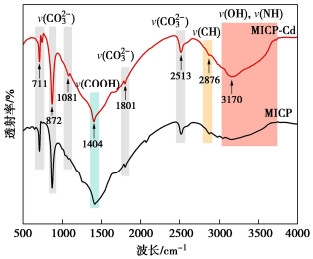
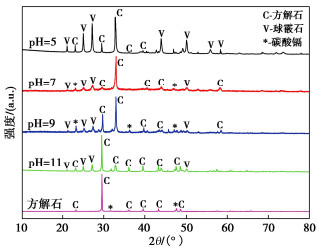
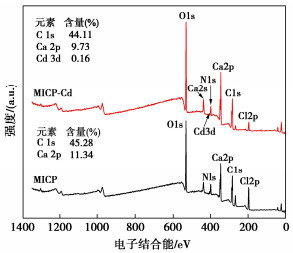
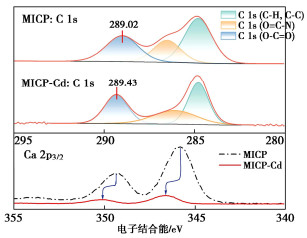
Effects of pH on MICP curing of cadmium-contaminated tailings
-
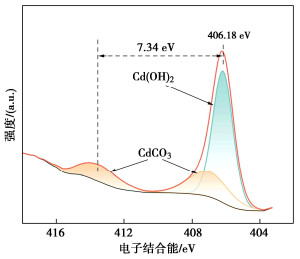
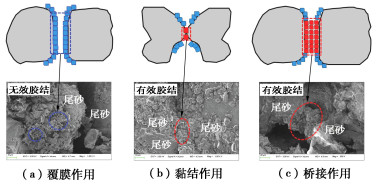
摘要: 微生物诱导碳酸钙沉淀(MICP)方法是一种生态友好、可持续的污染土固化与修复技术,而对于MICP在不同土壤pH值环境下处理重金属污染土影响的研究较少。利用巴氏芽孢杆菌ATCC 11859分别在pH值为5,7,9,11条件下进行了MICP修复Cd溶液试验、Cd污染尾矿固化修复试验。结果表明,水溶液修复试验中,在pH为5~11范围内均能有效钝化Cd2+,当pH值为9时对Cd2+钝化效果最佳;尾矿固化试验中,酸性淋溶液会加快尾矿中的Cd2+浸出,MICP主要将尾矿中Cd的酸可溶态转化为可还原态和残渣态,且钝化效果与碳酸盐生成率呈正相关关系。通过对生物矿化沉淀的FTIR、XRD、XPS和SEM-EDS分析表征,揭示了MICP钝化重金属和固化尾矿的机理:MICP通过生物吸附和碳酸钙的吸附、离子交换、共沉淀等作用完成对重金属离子的钝化,通过覆膜作用、黏结作用和桥接作用增大土颗粒内摩擦角和土粒间的黏聚力。
-
关键词:
- 微生物诱导碳酸钙沉淀 /
- pH值 /
- 固化修复污染土 /
- 镉 /
- 微观机理
Abstract: The microbial induced calcium carbonate precipitation (MICP) method is regarded as an environmentally sustainable approach for the solidification and remediation of contaminated soils. However, there are limited researches dedicated to understanding the impact of pH on the MICP treatment of heavy metals. In this study, the MICP remediation experiments involving cadmium (Cd) solution and Cd-contaminated tailings using Sporosarcina pasteurii ATCC 11859 at varying pH levels of 5, 7, 9, and 11 are conducted. The results indicate that in the aqueous solution tests, the effective immobilization of Cd2+ occurs across the pH range of 5 to 11. The optimal immobilization of Cd2+ is observed at a pH of 9. In contrast, during the consolidation and remediation tests on tailings, the introduction of acidic drenching solutions expedites the leaching of Cd2+ from the tailings. In this context, the MICP primarily transforms the acid-soluble fraction of Cd within the tailings into reducible and residual states. Importantly, the effectiveness of this passivation process exhibits a positive correlation with the rate of carbonate generation. The analytical characterization through FTIR, XRD, XPS and SEM-EDS of biomineralization precipitation offers insights into the mechanisms underlying the fixation of heavy metals by the MICP and the solidification of tailings. The MICP accomplishes the immobilization of heavy metal ions through a combination of biosorption and calcium carbonate adsorption, ion exchange and co-precipitation processes. Furthermore, the MICP enhances the friction angle of soil particles and promotes cohesion among them through coating, bonding and bridging effects. -
0. 引言
在水利水电工程当中,快速正确的对岩体完整性以及质量进行分级与评价是岩体开挖和加固支护设计、快速施工必不可少的条件,其作为重要课题一直被科研人员密切关注[1-5]。岩体质量主要受到岩体内部结构特性的影响,岩体完整性是重要作用的因素之一,在分类时需要考虑参数的不确定性。因此,如何对岩体完整性及岩体质量进行快速有效的划分,是岩土工程领域解决岩体安全稳定的一个重要问题。目前,岩体质量评价的方法主要有T评分[6]、Q系统[7]、地质强度指标(GSI)[8]、岩体地质力学分类(RMR)[9]等。这些指标结合了岩体本身的性质,在一定程度上反映了岩体的完整性,而这也对岩体质量的优劣起控制性作用。
针对岩体完整性判断问题,李明超等[10]基于岩体对穿声波波速数据,采用加权随机森林算法,提出了一种新的多尺度岩体完整性评价指标和相应的分析方法,该方法没有考虑节理及岩体岩性等情况。曹瑞琅等[11]通过提出钻进过程指数DPI,建立了DPI与RQD之间联系,为评价岩体完整性提供一种新指标,但钻孔中会忽略各向异性等问题。李清波等[12]基于边缘阈值分割钻孔图像的方法,对RQD进行自动分析,与波速测试及钻孔图像对比,提高RQD统计的准确性,但对于图像中反射差异不明显的结构面,还未能进行研究。Guo等[13]基于高清数字钻孔电视技术,提出了一种新的完整性评价方法,克服了RQD计算过程中岩心脱落和破碎问题的影响,在实际施工过程中,能够对岩体的完整性及裂隙的演化过程进行动态评价,但缺乏对结构面发育情况以及特征的研究。殷明伦等[14]通过统计调研大量工程数据,研究体积节理数Jv与岩体完整性系数Kv之间的关系,提出了考虑结构面类别的Jv表征Kv的修正方法,但理论上还是根据Kv对岩体完整性进行评价,并且在实际工程中Jv与Kv的关系较差。Song等[15]根据岩体完整性系数Kv与地质强度指标GSI关系,推导出Kv与RMR评分体系中某些项的关系,用这些指标进行预测Kv有着很高的相关性,其最终只是得到Kv值,是预测获取Kv值的另一途径,不能很好地进行岩体完整性评价。
实际工程中评价岩体质量时,常利用RQD、Kv、Jv中某一定量指标对岩体完整性进行分析,不能综合反映岩体特征,导致结果代表性差,与实际情况不符[16]。例如,RQD不能与岩块挂钩,具有多解性;Kv主要考虑的是地下水、高地应力、卸荷等影响;Jv仅能反映结构面的几何发育特征。
针对水利工程中岩体完整性评价问题,耦合多源勘察信息,通过采用熵权-云模型对工程中误判数据进行修正,并使用少数类过采样方法对岩体完整性分级中不均衡数据进行预处理,改进数据集结构,利用随机森林算法对岩体完整性进行分类预测;由于影响岩体完整性评价的因素较多(如节理、地质构造、风化程度以及地下水等),为避免单一指标误差大,减少人为判断的主观性,结合影响因素对实际情况进行具体分析,并对所提出的方法进行应用拓展,为工程岩体完整性分析提供新的方法。
1. 岩体完整性指标及多指标的融合
1.1 岩体质量指标RQD
岩体质量指标(rock quality designation,RQD)自Deere[17]提出后,便成为划分岩体结构特征的基本方法及重要参数。RQD常用岩芯钻探法进行获取,需要严格采用金刚石钻头、双层岩芯管钻进进行实现[18]。RQD为一定量指标,是某岩组中大于或等于10 cm的柱状岩芯的累计长度与某岩组钻探总进尺之比[19],以百分数表示:
RQD=A1A×100%, (1) 式中,A1为累计大于等于10 cm岩芯长度,A为单位岩芯钻孔总长度。
RQD计算方式便捷,同时作为裂隙间距以及密度的指标,可以很好地反映岩体的连通性、压缩性、渗透性等物理力学性质,能定量反映岩体的完整性。但是,传统的RQD计算方法没有考虑阈值的合理性,不能很好地体现岩体的尺寸效应和各向异性,没有涉及岩体中结构面的发育情况[20],难以反映岩体结构本身的复杂性,导致评价结果会与真实情况有一定出入。
1.2 岩体体积节理数Jv
岩体体积节理数Jv是国际岩石力学委员会推荐使用评价岩体完整性和岩体节理化程度的指标,Jv的定义为单位体积内包含节理的数目,工程中常常采用间距法及条数法进行计算,最后取二者均值得出该施工阶段或工程地质勘察阶段的情况。间距法公式为
Jv=(1S1+1S2+⋅⋅⋅+1Sn) (i=1,2,3,⋅⋅⋅,n), (2) 式中,Si为各组裂隙平均间距,i为裂隙数。
条数法计算方法如下:
Jv=N∑i=1niN×(1.3~1.5) (i=1,2,3,⋅⋅⋅,n), (3) 式中,ni为第i个单位测量面积内的节理条数,N为同级别岩体中的样本数,一般不少于10个。
获取Jv的方法简单快捷,并且该指标能很好反映结构面的组数、迹长、间距等岩体结构面的几何发育特征,可以定量评价岩体的完整性,然而其不能够反映结构面的结合程度,评价的结果依赖于专家的个人经验与能力,会产生一定的误差。
1.3 岩体完整性系数Kv
岩体完整性系数可以定量表达岩体的完整程度,Kv定义为岩体中纵波的传播速度与岩石中的传播速度的比值的平方,即
KV=(vpmvpr)2。 (4) 式中:vpm为岩体当中的纵波波速,纵波波速通常采用弹性波勘探技术进行收集;vpr为岩石当中的纵波波速。
岩体完整性系数Kv的大小反映了岩体相对于岩石的完整或破坏程度,常作为各种评价指标中的一项重要参数,通常Kv值小于1;当所测岩体处于高地应力[21]、试验测试方向以及岩体含水状态等[22]情况时,会出现Kv值大于1;当岩块从原有岩层取出时,产生卸荷效应,使波速发生变化,导致结果不准[23]。
1.4 多源数据耦合方法
目前,用于表征岩体完整性的指标较多[24],常用指标为岩体质量指标RQD、岩体完整性系数Kv以及岩体体积节理数Jv。GB50487—2008[6]及GB50287—2016[24]根据结构面组数及结构面间距确定,但只用Kv对岩体完整性进行定量评价;GB50287—2016[24]中可使用Jv及Kv作为岩体完整性的定量指标;3个规范对于Kv的评分范围皆是相同的,Jv采用GB/T50218—2014[25]中评分,具体见表 1;鲁布革、二滩水电站、《中小型水利水电工程地质勘察规范:SL55—93》[26]采用RQD对岩体完整性程度进行分析,但RQD评分范围不同,学者常用表 1中的数据进行研究[27]。
表 1 岩体完整性评价标准Table 1. Evaluation standards for rock mass integrity完整
程度完整 较完整 完整
性差较破碎 破碎 RQD/% 90~100 75~90 50~75 25~50 0~25 Jv/
(条·m-3)0~3 3~10 10~20 20~35 >35 Kv 0.75~1 0.55~0.75 0.35~0.55 0.15~0.35 0~0.15 因此,根据相关工程经验以及各种评价方法,将岩体按照完整程度划分为完整、较完整、完整性差、较破碎和破碎5个等级。相关岩体完整性评价标准如表 1所示。
工程数据中存在工程师误判的情况,因此利用熵权-云模型对数据进行修正,具体步骤如下:
(1)整理确定岩体完整性评价指标,并依据规范确定各级分类标准见表 1。
(2)将各指标进行归一化处理,并根据下式利用熵权法确定指标权重:
Hj=−m∑i=1kijlnkijlnm, (5) W=1−Hjn∑j=1(1−Hj)。 (6) 式中:Hj为信息熵;kij为无量纲化后的评价指标值;m为待评对象数量;n为每个待评对象包含的评价指标;W为个指标权重。
(3)通过各个指标的上下限,根据下式确定云模型的数字特征:
Ex=(Cmin+Cmax)/2 ,En=(Cmax−Cmin)/6 ,He=k 。} (7) 式中,Cmin与Cmax为某一岩体完整性等级范围的最小值与最大值,Ex为该范围内期望,En为该范围内熵,He为该范围内超熵,k为常数,取0.002。
(4)采用正向发生器生成不同指标对应的云模型,根据
Y=n∑i=1μiWi (8) 计算综合确定度Y,最后依据最大综合确定度原则,修正岩体完整性等级。
经过熵权-云模型预处理后,得到的数据评价结果与真实情况更加符合。该处理可以修正工程中判断误差较大部分,对于相邻评价等级则以工程师评价为准。
2. 岩体完整性智能评价方法
2.1 总体流程
针对单一指标评价岩体完整性的局限性,本文将耦合工程中常用评价3个指标RQD、Jv、Kv,综合评价岩体完整性,增加准确率。图 1为主要研究路线,主要包括:①计算工程中3个评价岩体完整性指标,利用熵权-云模型对工程数据修正;②利用SMOTE算法对少数类样本中数据进行均衡;③使用随机森林分别对原始样本数据及均衡后数据进行训练分类;④计算Kappa系数及绘制混淆矩阵,结合影响岩体完整性因子,对多工程进行评价分析。
2.2 SMOTE算法
合成少数类过采样技术(synthetic minority oversampling technique,SMOTE)[28]通常用于处理不平衡数据集。分类模型处理不平衡数据集时对少数类样本的分类效果通常较差,且当少数类样本远小于多数类样本时,预测精度易出现虚高现象。利用SMOTE方法对实际工程数据进行预处理,可使样本集达到均衡,进而优化评价结果。SMOTE算法的具体思路如下:
(1)假设某一少数类样本集为S={s1, s2, …, sa},a为少数类岩体完整性样本数量。对于少数类中每一个样本s,采用欧氏距离计算它到少数类样本集中所有样本的距离,得到其k近邻。
(2)根据样本不平衡比例,确定生成的样本数量占原少数类样本的百分比N%,对于每一个样本si(i=1, 2, …, a)从k近邻中随机选取b个样本数据,放入sample={s′1, s′2, …, s′b}集合当中,每个数据记为s′i,其中,0⩽b⩽a,1⩽l⩽b。
(3)记采样倍数c=int(N/100),取(0,1)随机数r=rand(0,1),依据式(9)生成新的样本。
Sj=si+r(s′i−si) (j=1,2,3,⋅⋅⋅,c)。 (9) (4)重复(1)~(3)步骤合成其他少数类样本。
2.3 随机森林算法
随机森林算法(random forest,RF)是由Breiman[29]提出的一种分类算法,其有着简单、抗噪能力强、性能稳定等优点,被广泛地运用于各种领域数据处理中。RF模型算法的思路如下:
(1)设总集合为W,采用Bootstrap自助法随机有放回的选取训练样本,形成k个子训练集{Ni}(i=1, 2, 3, …, k),每一个训练样本为一棵CART决策树。
(2)k棵CART树组成了随机森林,每棵分类树的子节点进行分裂时,会从M个特征变量中随机选取m个变量(0<m≤M),作为每棵CART训练模型的指标。
(3)重复步骤(2),构建出随机森林中所有子树。
(4)将训练集导入构建好的RF模型当中,利用投票选举的方式进行统计,将票数最多的一类作为最终的预测结果。
RF算法在使用时通过Gini值作为切分节点的标准,Gini值反映了每个特征在每个CART树上做了多大的贡献,最后取均值,得出每个特征的贡献大小。RF预测模型的准确率跟CART树的数量以及最大深度有关,合理控制RF模型的参数可以使模型达到最优。
2.4 分类模型评价指标
岩体完整性预测模型通常为多分类模型可利用敏感性(sensitivity)、F1指标(F1-measure)等指标进行分析,但其步骤繁琐,因此本文采用混淆矩阵以及Kappa系数进行分析。混淆矩阵是一种图像精度评价,将实际值与预测值显示在同一个n行n列的矩阵中,效果直观简单。Kappa系数是基于混淆矩阵的一种统计学评估方法,实际工程中取值为[0,1],Kappa系数越高,代表模型分类准确度越高,
Pe=k∑i=1minik∑i=1mi (i=1,2,3,⋅⋅⋅,k), (10) K=P0−Pe1−Pe。 (11) 式中:k为类别总数;mi为第i类真实样本数;ni为第i类预测出来的样本数;P0为总体分类精度。
一致等级情况如表 2所示。
表 2 Kappa系数一致等级表Table 2. Scales of Kappa coefficient系数 0~0.2 0.2~0.4 0.4~0.6 0.6~0.8 0.8~1.0 一致等级 极低 一般 中等 高度 几乎完全一致 3. 工程应用
3.1 平硐勘查数据预处理与模型训练
为了验证模型的有效性,先依据某水电站坝址平硐勘探数据(记为工程1),分析模型在岩体完整性评价中的适用情况,再利用另一水利工程坝址勘探平硐数据(记为工程2)进行有效性验证,两工程数据均使用1.4节中熵权-云模型流程进行修正。工程1河谷狭窄,两岸陡峻,坡顶行对高差最多可达1000~1700 m,坡度可达30°~90°。其中数据包括9个勘探线,27个平硐,共被划分为118个平硐岩体完整性分段,岩体岩性以大理岩、粉砂质板岩为主。
采用RQD,Jv,Kv 3个常用评价岩体完整性指标进行模型训练及验证。Jv在计算时,式(3)中系数为1.3~1.5,因此取二者系数均值1.4,在与式(2)计算结果取均值;Kv计算时岩体中纵波波速选用对穿声波波速,少数缺省值利用插值进行计算。表 3列举出部分典型的实际勘探数据。
表 3 工程岩体多源勘察数据Table 3. Multi-source survey data of engineering rock mass序号 平硐 桩号 RQD/% Jv/(条·m-3) Kv 应力分带 岩体结构类型 岩体嵌合程度 完整性 1 PD01 220—250 99 6.0 0.85 集中带 块状结构 紧密 完整(1) 2 PD04 130—175 63 12.9 0.37 过渡带 次块状结构 较紧密 完整性差(3) 3 PD05 146.7—188 55 7.3 0.87 平稳带 镶嵌碎裂结构 较紧密 较完整(2) 4 PD07 70—160 80 5.5 0.88 过渡带 中厚层状结构 较紧密 较完整(2) 5 PD12 37—75 29 21.0 0.17 弱卸荷带 层状破碎结构 松弛 较破碎(4) 6 PD30 17—65 51 16.8 0.53 释放带 破碎结构 较松驰 完整性差(3) 工程1包含完整分段数据9个,较完整分段数据45个,完整性差分段数据54个,较破碎分段数据10个,采用的训练集与测试集比例为7∶3。由于4个分段数据比例不均衡,先利用SMOTE算法对完整数据与较破碎进行扩充,使得4个分段数据基本上相等,再使用RF算法对预处理后的数据进行调参训练,得到最优时最大树深为7,决策树数目为50,其余参数为默认值。同时,将未采用SMOTE算法处理的数据也进行训练,作为对比。
3.2 完整性评价与分析
如表 4所示,可以看出完整、较破碎两个少数类样本经过均衡调节处理后,准确率有所上升,分析数据可知,仅利用RF模型会将完整性岩体分类成较完整岩体,将较破碎岩体分类成较破碎岩体,使得与实际情况更加不符。而提前用SMOTE算法处理后再进行分类,整体准确率从83.3%提升到94.4%,提升明显。
表 4 两种模型输出结果与岩体完整性对应关系Table 4. Correspondence between output results of two models and rock mass integrity岩体完整性 两种机器学习模型测试准确率/% RF模型 SMOTE-RF模型 完整 33.3 66.7 较完整 92.3 92.3 完整性差 88.2 100.0 较破碎 66.7 100.0 总准确率 83.3 94.4 SMOTE-RF模型与RF模型K值分别为0.91,0.73,两者混淆矩阵如图 2,3所示。说明经过预处理后的数据,预测模型更加可靠,且少数类样本预测概率有所提升。
如表 5所示,为SMOTE-RF模型训练结果与实际情况不符分段。根据影响岩体完整性节理、岩性、卸荷、构造、风化、岩脉等因素对不符合分段进行简要分析。
表 5 工程岩体分段评价结果Table 5. Sectional evaluation results of engineering rock mass序号 平硐 桩号 野外勘探岩体完整性评价 SMOTE-RF模型岩体完整性评价 1 PD01 250—301.5 完整(1) 较完整(2) 2 PD46 190—215 较完整(2) 完整性差(3) PD01位于Ⅰ线右岸抗力体,硐深301.5 m,硐口高程1656.09 m。RQD、Jv、Kv值为96.3,9.25,0.96。岩体结构类型为镶嵌碎裂结构,以厚层角砾状大理岩为主,局部夹绿片岩透镜体,岩体整体处于微风化,局部弱风化,沿NWW向溶蚀裂隙发育,间距0.2~0.5 m,沿#114、#117溶蚀张开1~3 cm。250~301.5 m之间有4组规则裂隙,裂隙发育特征如表 6所示,说明该分段岩体裂隙发育,而RQD值没有考虑裂隙的发育情况,导致数值过高,与真实情况存在一定出入。
表 6 裂隙发育特征Table 6. Characteristics of fracture development组别 规则裂隙产状 裂隙线平均
间距统计(5 m×2 m)规则裂隙延伸长度 张开度/mm 粗糙度 充填物类型 主要结构面类型 地下水 1 N40-60°E/SE∠65-80° 一般 > 1,个别0.1~0.5 一般1~3 0~2 平糙 少许钙膜 节理裂隙 干燥 2 N30-50°E/NW∠25-35° 薄—中层 > 2 0~2 起滑 绿泥石膜 节理裂隙 湿润 3 N5-20°W/SW∠50-70° 零星 1~3 0~5 起糙 不连续泥膜、岩屑 结构面 湿润 4 N30-50°W/NE∠25-30° 零星 > 1 0~2 起糙 不连续泥膜、岩屑 结构面 湿润 另外,岩块嵌合较紧密,应力分带为集中-平稳带,存在地下水,如图 4中蓝色虚线框所示,在250~260 m,局部张开0.5~2 cm线状水,259 m附近处有滴水存在;285 m附近岩体湿润;296 m处为绿片岩与大理岩接触面,有连续滴水-流水,层面起伏,这些会导致测得的纵波波速偏高[30],从而导致Kv值变高。
表 7 该岩体分段三组构造带基本情况与特征Table 7. Basic situations and characteristics of three groups of structural zones in rock mass编号 构造类型 产状 起伏情况 充填物 力学性质 其他特征 f46-1 切层错动带 N25E/SE∠80 起伏 灰黄色压碎岩 压性 上游壁与顶拱接触处见一长25 cm,宽
6 cm,深60 cm的溶洞f46-3 切层错动带 N75E/NW∠70 起伏 黑色压碎岩 压性 含土白色方解石粉沫,局部软化夹泥;上
游壁见宽40~80 cm影响带,逆断层f46-4 切层错动带 N20E/SE∠25 微起伏 灰黑色角砾岩及
压碎岩压扭性 局部软化,泥化;下盘见宽1.2 m破碎岩,
破碎结构,上盘湿润因此,虽然该分段的RQD与Kv值较高,但Jv值处于较完整分段,并且接近于完整性差分段,岩体完整性有待商榷。
PD46位于Ⅵ线左岸抗力体下游部位,硐口高程为1818.1 m,硐深250.3 m。RQD,Jv,Kv值为74.6,9.13,0.56。如表 7所示,该段受f46-1、f46-3、f46-4三组构造带影响,断层两侧同倾向裂隙发育,岩体较松弛、完整性差,在顶拱213.2 m处存在一平硐层间挤压带,风化较强烈,裂隙主要发育3组,裂面普遍中锈,充填不连续钙膜,岩块嵌合较松弛。虽然整体野外评价为较完整,但是此分段存在各种不良地质体,且三者值处于较完整与完整性差边界处,因此需要酌情考虑此处岩体完整性,避免施工造成损失。
3.3 SMOTE-RF模型工程应用拓展
为进一步验证方法模型的有效性及适用性,利用工程2中的数据对工程1所训练的模型进行研究。工程2距离区域性断裂较近,区域内岩体主要由燕山早期侵入岩-花岗闪长岩组成,地质构造相对简单。其中数据包括7个勘探线,16个平硐,共被划分为87个平硐岩体完整性分段,岩性以花岗岩为主,存在砂岩;包含完整,较完整、完整性差和较破碎分段数据分别为20,34,25,2个,由于较破碎岩体分段数据较少,因此选择前3个分段进行验证,训练集与测试集比例为7∶3,保持两种模型的各项参数,得出模型分类的准确率如表 8所示,可以看出分类为完整以及完整性差的岩体准确率有所上升,说明SMOTE-RF模型更优,更加符合实际情况。
表 8 两种模型输出结果与岩体完整性对应关系Table 8. Correspondence between output results of two models and rock mass integrity岩体完整性 两种机器学习模型测试准确率/% RF模型 SMOTE-RF模型 完整 66.7 83.3 较完整 100.0 100.0 完整性差 62.5 87.5 总准确率 79.2 91.7 两种模型的K值分别为0.87,0.67,二者混淆矩阵如图 5,6所示。可以看出,完整与完整性差少数类分段在SMOTE数据处理过后,预测效果更好,可以进一步扩充该类中的关键特征。
SMOTE-RF模型评价与实际不符情况如表 9所示。包括PD102与PD107中两个分段,将完整以及完整性差岩体均预测成了较完整岩体,先对这两分段进行分析。
表 9 工程岩体分段评价结果Table 9. Sectional evaluation results of engineering rock mass序号 平硐 桩号 野外勘探岩体完整性评价 SMOTE-RF模型岩体完整性评价 1 PD102 64—85 完整(1) 较完整(2) 2 PD107 25—38 完整性差(3) 较完整(2) PD102位于Ⅲ勘探线线左岸下坝址,硐深152 m,硐口高程2263 m,RQD,Jv,Kv分别为94,3.7和0.74。该段岩性为花岗闪长岩,有2组裂隙发育,间距20~30 cm,中度发育,岩体新鲜,较紧密,次块-镶嵌结构,岩体无卸荷,存在两条岩脉,弱风化,较坚硬,与围岩焊熔接触,湿润,岩脉其它特征描述表如表 10所示,两组岩脉如图 7所示,因此该段为较完整岩体更合适。
表 10 工程岩体岩脉特征描述表Table 10. Description of dike characteristics of engineering rock mass编号 桩号 产状 宽度/cm 颜色 特征 完整性 蚀变程度 Ym102~5 左72顶72.8右75 N60°E/NW∠80° 30~60 浅黄色 发育断层f102~5及宽
2~4 cm的石英脉,石英
脉局部宽9 cm完整性差 蚀变不明显 Ym102~6 左78.3~79.4顶78.5~79.1右81~81.6 N60°E/NW∠60° 60~110 灰白—浅黄色 宽2~3 cm的石英脉,
断续延伸较完整 上盘局部蚀变3~5 cm PD107位于下横Ⅳ线左岸,硐深151 m,硐口高程为2263 m。RQD,Jv,Kv分别为86,9.85,0.32。该段岩体为花岗闪长岩,裂隙发育2组,间距30~50 cm,中度发育;该分段岩体质量等级为Ⅲ2,弱风化,弱卸荷,较紧密,次块-块状结构,但此处岩体干燥,岩体含水率低,导致对穿声波波速值较小,因此将此处岩体完整性评定为较完整更加合理,但具体施工时还需要依据现场情况采取相应措施。
3.4 讨论
依据表 1利用常用传统指标RQD,Kv,Jv对两个工程测试集中数据分别进行岩体完整性评价,三者评价及耦合勘探信息情况如图 8所示。利用单一指标对岩体分段进行评价时,工程1与工程2中的依据单指标RQD,Kv,Jv评价准确率分别为75%,50%,83.3%以及50%,41.7%,62.5%,而经过少数类样本生成后,利用RF进行预测的准确率分别为94.4%,91.7%。
可以看出,单一指标评价方法只能从部分角度反映岩体的完整程度,较为片面。耦合多源勘察信息指标可以减少因单一指标的造成的误差,增加岩体完整性评价的准确率,有助于工程师在进行野外勘探时判断岩体的分类情况。
4. 结论
针对传统工程岩体完整性评价时仅利用单一指标导致的评价精度差、准确率低等问题,提出了耦合多源勘察数据的SMOTE-RF模型,对具体工程岩体完整性数据不均衡分段进行定量评价分析,得到以下3点结论。
(1)运用熵权-云模型方法对数据进行预处理,可以检测出工程中误判数据。通过耦合多源勘探信息方法以及对比传统的方法,不难看出利用该方法得出来的效果比传统方法好,可以减少因单一指标反映岩体情况不周导致的效果差、准确率较低等问题,可以有效减少主观评价时存在的问题。
(2)对于工程中原始岩体完整性分段数据比例不均衡问题,利用SMOTE算法对少数类样本进行过采样处理,使训练集数据分布均衡,改善数据结构,进而采用RF机器学习模型对工程岩体完整性分段数据进行分类预测与验证,准确率得到较大提升。对其他工程进行了应用拓展,进一步说明了SMOTE-RF模型的有效性,且相较RF模型岩体完整性预测结果与实际情况更加符合,证明了该模型的有效性和普适性。
(3)预测结果问题分析:①样本数据量一直都是影响分类算法中的重要因素,少数类样本特征信息包含不全面,导致将少数类分类为多数类中;②当数据处于边界位置时,例如PD46中RQD,Jv,Kv值为74.6,9.13,0.56,依据表 1分类为较完整岩体,但分类为完整性差岩体;③当勘探参数跨度较大时,例如PD107中RQD,Jv,Kv值分别为86,9.85,0.32,跨度较大原因为各参数考虑因素不同,因此可能导致分类不准。
针对上述存在预测结果的问题,尚需进一步深入研究:结合其他工程数据,增加数据量,以减少单工程中分段特征相似情况,对于边界数据能更好考虑;将机器学习算法与评价标准进一步融合,提升模型方法的准确率。
-
表 1 尾矿砂主要化学成分
Table 1 Main components of tailings sand
(%) K2O TiO2 Na2O CaO MgO Al2O3 Fe2O3 SiO2 1.42 1.73 5.02 5.55 5.72 15.07 19.95 43.85 -
[1] TIWARI N, SATYAM N, SHARMA M. Micro-mechanical performance evaluation of expansive soil biotreated with indigenous bacteria using MICP method[J]. Scientific Reports, 2021, 11(1): 10324. doi: 10.1038/s41598-021-89687-2
[2] KUMARI D, PAN X L, LEE D J, et al. Immobilization of cadmium in soil by microbially induced carbonate precipitation with Exiguobacterium undae at low temperature[J]. International Biodeterioration & Biodegradation, 2014, 94: 98-102.
[3] LIAO Z S, WU S J, XIE H, et al. Effect of phosphate on cadmium immobilized by microbial-induced carbonate precipitation: Mobilization or immobilization?[J]. Journal of Hazardous Materials, 2023, 443: 130242. doi: 10.1016/j.jhazmat.2022.130242
[4] ZENG Y, CHEN Z Z, LYU Q Y, et al. Microbiologically induced calcite precipitation for in situ stabilization of heavy metals contributes to land application of sewage sludge[J]. Journal of Hazardous Materials, 2023, 441: 129866. doi: 10.1016/j.jhazmat.2022.129866
[5] LAUCHNOR E G, TOPP D M, PARKER A E, et al. Whole cell kinetics of ureolysis by Sporosarcina pasteurii[J]. Journal of Applied Microbiology, 2015, 118(6): 1321-1332. doi: 10.1111/jam.12804
[6] YI H H, ZHENG T W, JIA Z R, et al. Study on the influencing factors and mechanism of calcium carbonate precipitation induced by urease bacteria[J]. Journal of Crystal Growth, 2021, 564: 126113. doi: 10.1016/j.jcrysgro.2021.126113
[7] DONG Y R, GAO Z Q, WANG D, et al. Optimization of growth conditions and biological cementation effect of Sporosarcina pasteurii[J]. Construction and Building Materials, 2023, 395: 132288. doi: 10.1016/j.conbuildmat.2023.132288
[8] ZEHNER J, RØYNE A, SIKORSKI P. Calcite seed-assisted microbial induced carbonate precipitation (MICP)[J]. PLoS One, 2021, 16(2): e0240763. doi: 10.1371/journal.pone.0240763
[9] ZHAO Y, YAO J, YUAN Z M, et al. Bioremediation of Cd by strain GZ-22 isolated from mine soil based on biosorption and microbially induced carbonate precipitation[J]. Environmental Science and Pollution Research International, 2017, 24(1): 372-380. doi: 10.1007/s11356-016-7810-y
[10] SUN Y S, LV J G, TUO Y, et al. Experimental study on the influence of different factors on the mechanical properties of a soil-rock mixture solidified by micro-organisms[J]. Materials, 2022, 15(20): 7394. doi: 10.3390/ma15207394
[11] TANG C S, YIN L Y, JIANG N J, et al. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: a review[J]. Environmental Earth Sciences, 2020, 79(5): 94. doi: 10.1007/s12665-020-8840-9
[12] LIU J, LI G, LI X A. Geotechnical engineering properties of soils solidified by microbially induced CaCO3 precipitation (MICP)[J]. Advances in Civil Engineering, 2021, 2021: 6683930. doi: 10.1155/2021/6683930
[13] 公路土工试验规程: JTG 3430—2020[S]. 北京: 人民交通出版社, 2020. Test Methods of Soils for Highway Engineering: JTG 3430—2020[S]. Beijing: China Communications Press, 2020. (in Chinese)
[14] 孙潇昊, 缪林昌, 童天志, 等. 砂土微生物固化过程中尿素的影响研究[J]. 岩土工程学报, 2018, 40(5): 939-944. doi: 10.11779/CJGE201805020 SUN Xiaohao, MIAO Linchang, TONG Tianzhi, et al. Effect of methods of adding urea in culture media on sand solidification tests[J]. Chinese Journal of Geotechnical Engineering, 2018, 40(5): 939-944. (in Chinese) doi: 10.11779/CJGE201805020
[15] WU J Z, LI Z T, HUANG D, et al. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils[J]. Journal of Hazardous Materials, 2020, 387: 122010. doi: 10.1016/j.jhazmat.2019.122010
[16] RATHI M, YOGALAKSHMI K N. Brevundimonas diminuta MYS6 associated Helianthus annuus L for enhanced copper phytoremediation[J]. Chemosphere, 2021, 263: 128195. doi: 10.1016/j.chemosphere.2020.128195
[17] ALI A, LI M, SU J F, et al. Brevundimonas diminuta isolated from mines polluted soil immobilized cadmium (Cd2+) and zinc (Zn2+) through calcium carbonate precipitation: Microscopic and spectroscopic investigations[J]. The Science of the Total Environment, 2022, 813: 152668. doi: 10.1016/j.scitotenv.2021.152668
[18] FAN Y R, ZHENG C L, HUO A D, et al. Investigating the binding properties between antimony(V) and dissolved organic matter (DOM) under different pH conditions during the soil sorption process using fluorescence and FTIR spectroscopy[J]. Ecotoxicology and Environmental Safety, 2019, 181: 34-42. doi: 10.1016/j.ecoenv.2019.05.076
[19] ZHENG T W, QIAN C X. Influencing factors and formation mechanism of CaCO3 precipitation induced by microbial carbonic anhydrase[J]. Process Biochemistry, 2020, 91: 271-281. doi: 10.1016/j.procbio.2019.12.018
[20] HAN L J, LI J S, XUE Q, et al. Bacterial-induced mineralization (BIM) for soil solidification and heavy metal stabilization: a critical review[J]. Science of the Total Environment, 2020, 746: 140967. doi: 10.1016/j.scitotenv.2020.140967
[21] FENG Z Y, ZHAO Y X, ZENG W L, et al. Using microbial carbonate precipitation to improve the properties of recycled fine aggregate and mortar[J]. Construction and Building Materials, 2020, 230: 116949. doi: 10.1016/j.conbuildmat.2019.116949
[22] KEYKHA H A, ASADI A, ZAREIAN M. Environmental factors affecting the compressive strength of microbiologically induced calcite precipitation-treated soil[J]. Geomicrobiology Journal, 2017, 34(10): 889-894. doi: 10.1080/01490451.2017.1291772
[23] ACHAL V, PAN X L, FU Q L, et al. Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli[J]. Journal of Hazardous Materials, 2012, 201: 178-184.
[24] CHONG K Y, CHIA C H, ZAKARIA S. Polymorphs calcium carbonate on temperature reaction[C]// AIP Conference Proceedings. Selangor, Malaysia. AIP Publishing LLC, 2014: 52-56.
[25] QIAN X Y, FANG C L, HUANG M S, et al. Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil[J]. Journal of Cleaner Production, 2017, 164: 198-208. doi: 10.1016/j.jclepro.2017.06.195
[26] 曾勇, 陈泽智, 杜亚玲, 等. 产脲酶菌株Sporosarcina ureilytica ML-2诱导方解石沉淀矿化Pb(Ⅱ)、Cd(Ⅱ)和Cr(Ⅵ)研究[J]. 环境科学学报, 2022, 42(5): 148-159. ZENG Yong, CHEN Zezhi, DU Yaling, et al. The mineralization study of Pb(II), Cd(II) and Cr(VI) by induced calcite precipitation by urease producing strain Sporosarcina ureilytica ML-2[J]. Acta Scientiae Circumstantiae, 2022, 42(5): 148-159. (in Chinese)
[27] ZHU X J, LI W L, ZHAN L, et al. The large-scale process of microbial carbonate precipitation for nickel remediation from an industrial soil[J]. Environmental Pollution, 2016, 219: 149-155. doi: 10.1016/j.envpol.2016.10.047
[28] OLIVEIRA R C, HAMMER P, GUIBAL E, et al. Characterization of metal–biomass interactions in the lanthanum(III) biosorption on Sargassum sp using SEM/EDX, FTIR, and XPS: Preliminary studies[J]. Chemical Engineering Journal, 2014, 239: 381-391. doi: 10.1016/j.cej.2013.11.042
[29] WANG M L, WU S J, GUO J N, et al. Immobilization of cadmium by hydroxyapatite converted from microbial precipitated calcite[J]. Journal of Hazardous Materials, 2019, 366: 684-693. doi: 10.1016/j.jhazmat.2018.12.049
[30] MA L, PANG A P, LUO Y S, et al. Beneficial factors for biomineralization by ureolytic bacterium Sporosarcina pasteurii[J]. Microbial Cell Factories, 2020, 19(1): 12. doi: 10.1186/s12934-020-1281-z
[31] SHENG M P, PENG D H, LUO S H, et al. Micro-dynamic process of cadmium removal by microbial induced carbonate precipitation[J]. Environmental Pollution, 2022, 308: 119585. doi: 10.1016/j.envpol.2022.119585
[32] HAN Y S, HADIKO G, FUJI M, et al. Crystallization and transformation of vaterite at controlled pH[J]. Journal of Crystal Growth, 2006, 289(1): 269-274. doi: 10.1016/j.jcrysgro.2005.11.011
[33] 成亮, 钱春香, 王瑞兴, 等. 碳酸岩矿化菌诱导碳酸钙晶体形成机理研究[J]. 化学学报, 2007, 65(19): 2133-2138. doi: 10.3321/j.issn:0567-7351.2007.19.008 CHENG Liang, QIAN Chunxiang, WANG Ruixing, et al. Study on the mechanism of calcium carbonate formation induced by carbonate-mineralization microbe[J]. Acta chimica sinica, 2007, 65(19): 2133-2138. (in Chinese) doi: 10.3321/j.issn:0567-7351.2007.19.008
[34] LÜ C, TANG C S, ZHU C, et al. Environmental dependence of microbially induced calcium carbonate crystal precipitations: experimental evidence and insights[J]. Journal of Geotechnical and Geoenvironmental Engineering, 2022, 148(7): 04022050. doi: 10.1061/(ASCE)GT.1943-5606.0002827
[35] CHADA V G R, HAUSNER D B, STRONGIN D R, et al. Divalent Cd and Pb uptake on calcite{10ˉ14}cleavage faces: an XPS and AFM study[J]. Journal of Colloid and Interface Science, 2005, 288(2): 350-360. doi: 10.1016/j.jcis.2005.03.018
[36] ZHOU X L, LIU W Z, ZHANG J, et al. Biogenic calcium carbonate with hierarchical organic-inorganic composite structure enhancing the removal of Pb(II) from wastewater[J]. ACS Applied Materials & Interfaces, 2017, 9(41): 35785-35793.
[37] GODELITSAS A, ASTILLEROS J M, HALLAM K, et al. Interaction of calcium carbonates with lead in aqueous solutions[J]. Environmental Science & Technology, 2003, 37(15): 3351-3360.
[38] DEJONG J, MORTENSEN B M, MARTINEZ B C, et al. Bio-mediated soil improvement[J]. Ecological Engineering, 2010, 36(2): 197-210. doi: 10.1016/j.ecoleng.2008.12.029
[39] DEJONG J T, FRITZGES M B, NÜSSLEIN K. Microbially induced cementation to control sand response to undrained shear[J]. Journal of Geotechnical and Geoenvironmental Engineering, 2006, 132(11): 1381-1392. doi: 10.1061/(ASCE)1090-0241(2006)132:11(1381)
[40] DEJONG J T, SOGA K, KAVAZANJIAN E, et al. Biogeochemical processes and geotechnical applications: progress, opportunities and challenges[J]. Géotechnique, 2013, 63(4): 287-301. doi: 10.1680/geot.SIP13.P.017
[41] CUI M J, ZHENG J J, ZHANG R J, et al. Influence of cementation level on the strength behaviour of bio-cemented sand[J]. Acta Geotechnica, 2017, 12(5): 971-986. doi: 10.1007/s11440-017-0574-9
[42] 张茜, 叶为民, 刘樟荣, 等. 基于生物诱导碳酸钙沉淀的土体固化研究进展[J]. 岩土力学, 2022, 43(2): 345-357. ZHANG Qian, YE Weimin, LIU Zhangrong, et al. Advances in soil cementation by biologically induced calcium carbonate precipitation[J]. Rock and Soil Mechanics, 2022, 43(2): 345-357. (in Chinese)
-
期刊类型引用(1)
1. 唐小东. 基于三维地质模型的路桥工程特殊地质岩体勘察方法研究. 中国高新科技. 2024(07): 39-41 .  百度学术
百度学术
其他类型引用(0)
-
其他相关附件



 下载:
下载: